


Our laboratory develops and applies new approaches based on synthetic organic chemistry to chemical and biological discovery. Over the past several years we investigated two new lines of research. The first aim is to synthesize biologically important natural products. Total synthesis of these products enables not only steady supply of rare samples but also accelerates the chemical and biological investigations. Development of compounds with superior biological activity rather than natural products also lies in our goal. The second line seeks to develop new synthetic reactions. The reactions must be efficient and environmentally friendly. Our unique catalyst (yellow one in Figures) have been proved to be general and highly efficient. Moreover, our developed reaction with this catalyst allowed the high-yielding synthesis of the anti-influenza neuraminidase inhibitor (-)-oseltamivir (tamifulu) in "one-pot" sequences. We believe that our research contributes to human health and welfare as well as the scientific progress.
Direct Asymmetric Michael Reaction of α,β‐Unsaturated Aldehydes and Ketones Catalyzed by Two Secondary Amine Catalysts, Y. Hayashi, N. Umekubo, Angew. Chem. Int. Ed. 2018, 57, 1958-1962, DOI: 10.1002/anie.201710085.
Pot Economy in the Total Synthesis of Estradiol Methyl Ether by Using an Organocatalyst, Y. Hayashi, S. Koshino, K. Ojima, E. Kwon, Angew. Chem. Int. Ed. 2017, 56, 11812-11815, DOI: 10.1002/anie.201706046.
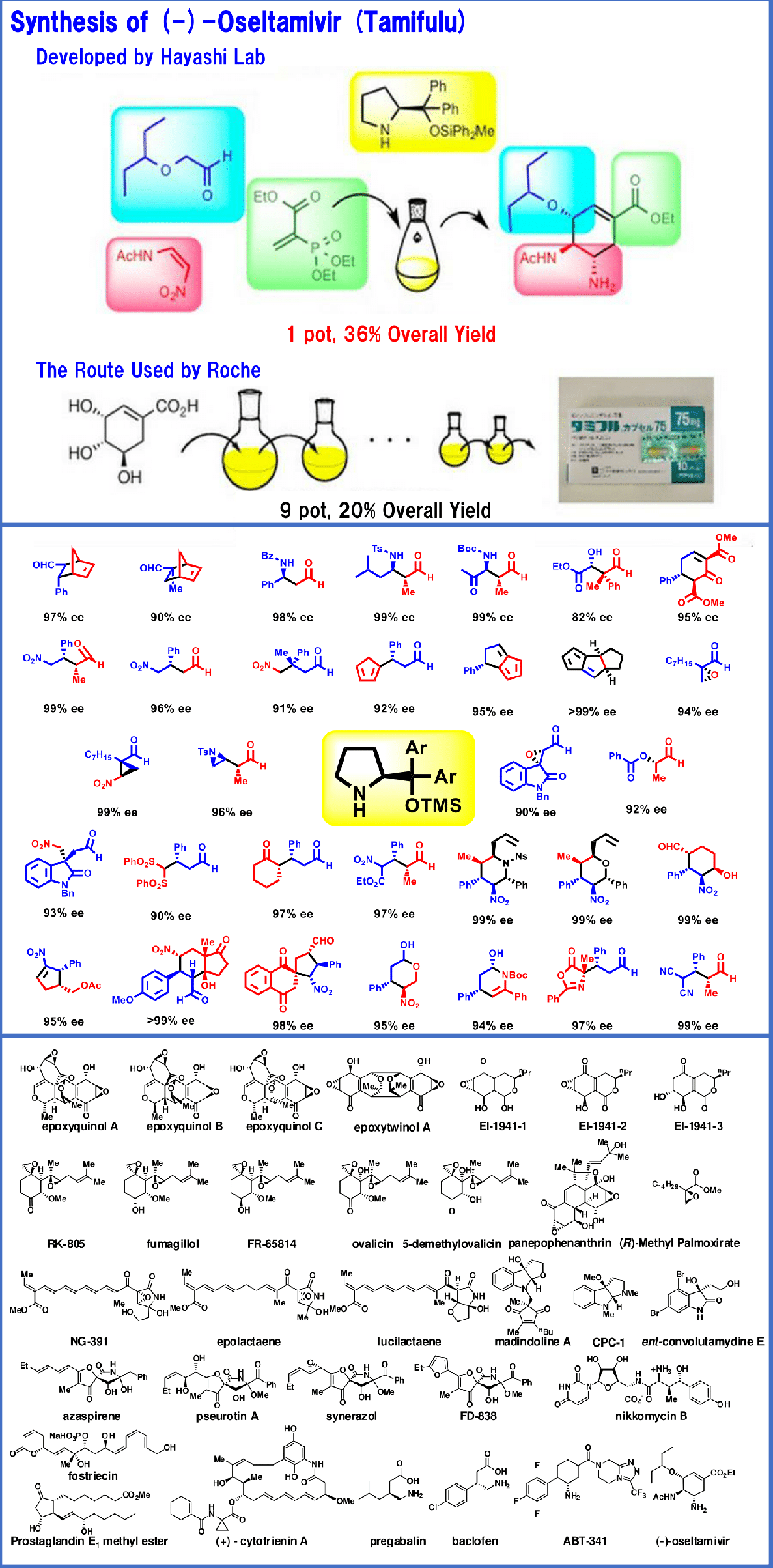
Multistep Continuous–Flow Synthesis of (-)-Oseltamivir, S. Ogasawara, Y. Hayashi, Synthesis, 2017, 49, 424-428, DOI: 10.1055/s-2016-0036-1588899.
Time Economical Total Synthesis of (−)-Oseltamivir, Y. Hayashi, S. Ogasawara, Org. Lett., 2016, 18, 3426-3429, DOI: 10.1021/acs.orglett.6b01595.
Pot economy and one-pot synthesis, Y. Hayashi, Chem. Sci., 2016, 7, 866-880, DOI: 10.1039/C5SC02913A.
“Please delete “/” after “@” in a mail address.”
Yujiro HAYASHI
(TEL +81-22-795-3554, Email: yhayashi@/m.tohoku.ac.jp)
Website: http://www.ykbsc.chem.tohoku.ac.jp/